Bange Lab - Research
As a protein’s nascent chain emerges from a translating ribosome, its N-terminus undergoes various modifications. Most common are the acetylation of the initiator N-terminal methionine (iMet), or its removal by methionine-aminopeptidases (MetAPs), exposing the second amino acid, often followed by sequence-specific acetylation of the newly generated N-terminus. Less common are N-terminal (Nt-) myristoylation, Nt-metylation, and Nt-arginylation. Our work focuses on N-terminal acetylation and its role in protein degradation/protein quality control and its interplay with metabolism.
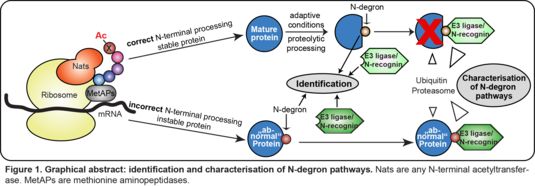
Identification and characterization of N-degron pathways
Regulated protein degradation controls levels of all short-lived proteins to ensure cellular homeostasis and protects cells from accumulating abnormal proteins. Dysfunctional degradation causes multiple pathological processes, spanning from neurodegenerative disorders to cancer. We are in interested in a specific subset of protein degradation pathways, so-called N-degron pathways, in which N-termini of proteins are recognized as degradation signals. The major players are E3 ligases, so-called N-recognins, which recognize N-degrons and target the proteins for proteasomal degradation. Although the first N-degron pathway (N-end-rule or Arg/N-degron pathway) has been discovered already in 1986, the identification and understanding of these pathways are still largely elusive. We aim to identify and characterize new N-degron pathways by using peptide pull-downs combined with mass spectrometry-based quantitative proteomics. (Figure 1)
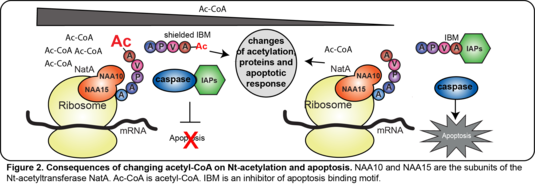
Investigation of the interplay between N-terminal acetylation and apoptosis
We recently identified the IAP (inhibitor of apoptosis proteins) E3 ligases, previously involved in apoptosis, as potential new N-recognins for proteins with an inhibitor of apoptosis binding motif which is present on ~5% of all proteins (Mueller et al, Science Advances, 2021). These N-degron motifs are in mammalian cells masked through N-terminal acetylation by the N-terminal acetyltransferase NatA. N-terminal acetylation uses acetyl-CoA as acetyl-group donor. Disturbance of N-terminal acetylation leads to the exposure of the N-degron motifs and subsequent apoptosis. We investigate by quantitative MS and biochemical assays how perturbations of acetyl-CoA metabolism affect N-terminal acetylation, protein levels, and IAP-dependent apoptotic responses in cancer and healthy cell lines. (Figure 2)

